Inquiry
Antibody Humanization
Antibody humanization is a process in biotechnology that involves taking a non-human antibody and modifying it to make it more similar to a human antibody. It is a key tool in the development of new and effective therapeutic antibodies.
Yurogen offers antibody humanization services for multiple species’ antibodies, including mouse, camelid, and rabbit monoclonal antibodies, to human antibodies. Combining the traditional CDR grafting and artificial intelligence-assisted analysis for hot spot prediction, mutation and removal, the final delivered humanized antibodies can achieve good balances among specificity, affinity, and immunogenicity. Yurogen has completed more than 20 antibody humanization projects. Our expertise guarantees a short turnaround time, a competitive price, and the best delivery.
Service Features
Customized Vaccination Strategy
Include DNA immunization, cell immunization and VLP immunization etc.
Physicochemical Analysis and Evaluation of Drugability
One stop analysis including expression levels, aggregation, binding affinity, and thermal stability etc.
Development across Multiple Platforms
Include the immunogenicity evaluation, druggability prediction, humanization, delineating CDR regions and characterization of humanized antibodies.
Procedure
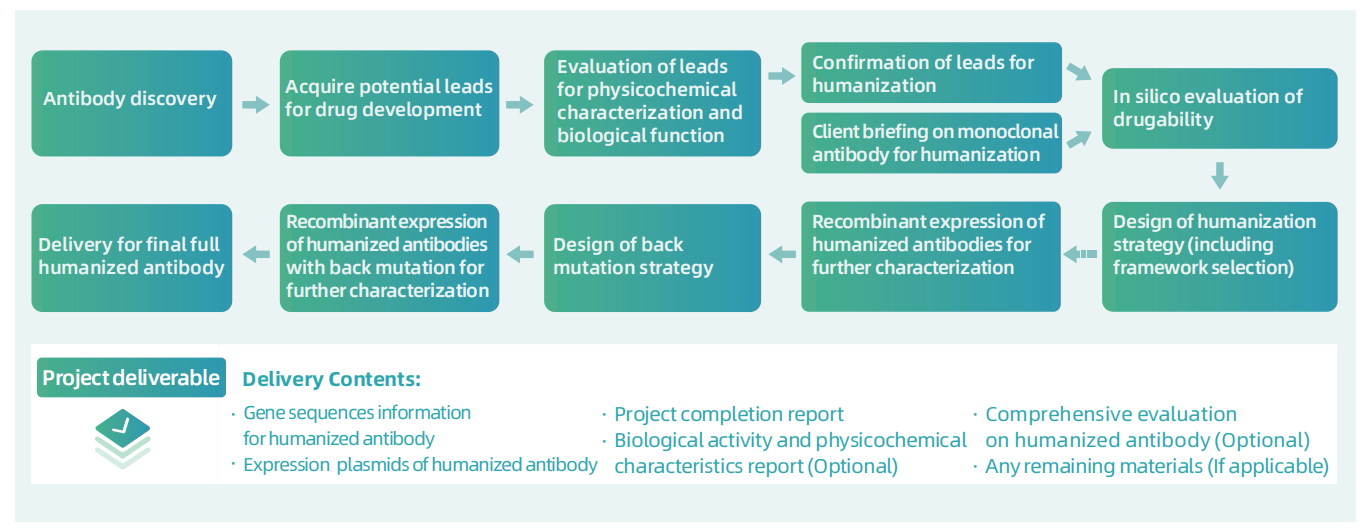
Workflow
| Phase | Service | Description | Timeline |
|---|---|---|---|
| Stage 0 | Expression and Production of Chimeric Antibodies |
| 1 week |
| Stage I | Humanization Sequence Analysis |
| 1 week |
| Stage II | CDR Transplantation |
| 1 week |
| Stage III | Affinity Maturation (optional) |
| 1 week |
| Stage IV | Humanized Antibody Expression |
| 1 week |
*Note: All the phases are around 5-6 weeks.
Yurogen SMab™ platform can directly deliver chimeric antibodies, and humanization services are available beginning with stage I onwards.
Yurogen SMab™ platform can directly deliver chimeric antibodies, and humanization services are available beginning with stage I onwards.
 English
English
